Another Medical Instrument Poses Risk Of Infection To Many Patients
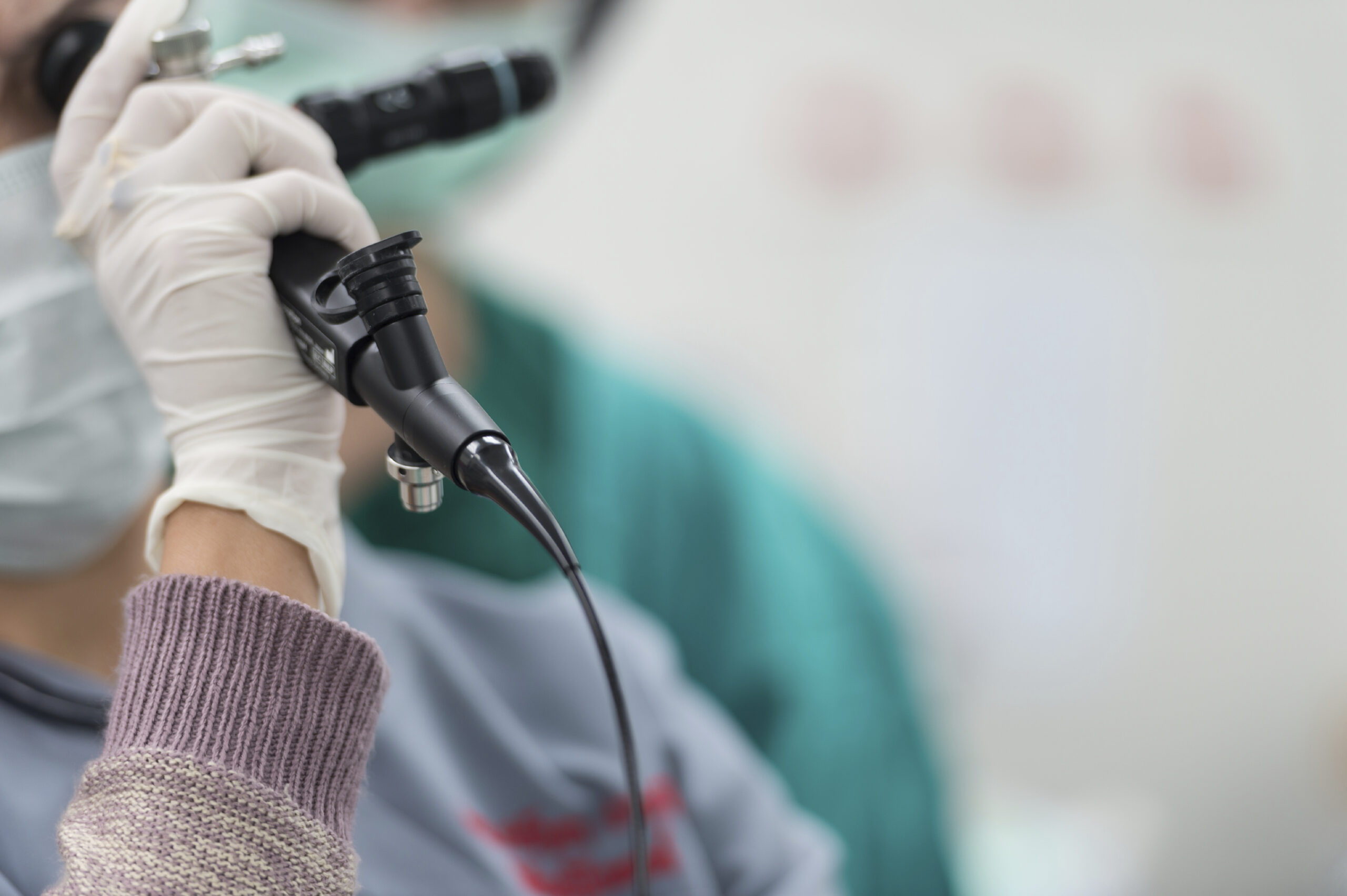
A few weeks back, we wrote about infections caused by contaminated duodenoscopes that have recently come to light. In the wake of these discoveries, other medical instruments have become the subject of scrutiny. Bronchoscopes are the latest FDA concern, a Bloomberg article reports.
A bronchoscope is a device that is threaded through a patient’s nose or mouth in order to examine his airway. The FDA says that they are used in about half a million procedures per year. Despite the chance of infection, bronchoscopes’ benefits outweigh the risks for most patients.
Between bronchoscopes and cystoscopes (a device threaded through the urethra), at least 32 contaminated scopes have affected 166 patients in the last two years. But this relatively small number may not be fully accurate. The FDA acknowledges underreporting of infection occurrences. It has warned manufacturers of the devices about failure to document adverse effects.
The spread of infection is not for lack of appropriate cleaning technique by medical professionals. These instruments are difficult to clean, as they contain hidden crevices and channels that harbor bacteria. Since the scopes are reusable, the bacteria is transferred from one patient to the next.
FDA records show 15 patients in Kentucky who were infected with B. cepacia. E. coli and P. aeruginosa were found in 22 patients in an unidentified state last year. The patients were treated by different doctors who used the same bronchoscope. Fortunately, these patients were all able to recover.
Warnings from the FDA most recently have advised healthcare providers and patients about the risk of infection. It is crucial for doctors to strictly follow manufacturers’ cleaning instructions for the scopes. Proper maintenance, storage, and quality control is critical, and any device with visible damage must be taken out of use immediately.
If you think you may have a medical malpractice claim, contact Paul M. da Costa, Esq. at Sarno da Costa D’Aniello Maceri Webb LLC. Call us today at 973-274-5200.


